How Many Core Electrons in Phosphorus
How many core electrons are there in a ground state phosphorus atom. How many are in Bismuth Bi core electron and valence electrons.
How Many Fully Filled And Partially Filled Electronic Shells Does Phosphorus Have Quora
The 10 remaining electrons from the first and second shells are core electrons.
. Remove the outer electrons to find the core electrons. In order to write the Phosphorus electron configuration we first need to know the number of electrons for the P atom there are 15 electrons. Out of those 5 are valence electrons.
That gives a total of 5 electrons so neutral phosphorus atoms have 5 valence electrons. There are 15 electrons in total there must be 5 electrons at the third energy level. And electrons equal to protons are located outside the nucleus.
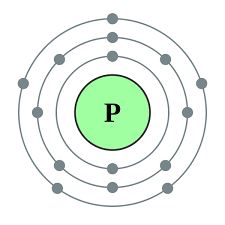
How many core electrons are in phosphorus. Therefore the order of the number of electrons in each shell of phosphorusP atom is 2 8 5. Phosphorus has atomic number 15 and is in group 5 and period 3 of the Periodic Table.
These are contained in the third energy level of the atom. The phosphorus atom has a total of 15 electrons so we have to put 15 electrons in orbitals. There are three unpaired electrons.
That gives a total of 5 electrons so neutral phosphorus atoms have 5 valence electrons. That means phosphorus has 10 core electrons. 495 4360 Views.
In the ground state the electronic configuration is 1s22s22p63s23p3. 31 15 16 so phosphorus has 16 neutrons. 1s2 2s2 2p6 3s2 3p3.
Two electrons can go into first shell eight in the second shell and it has five more in the third shell. What is the atom in order of increasing radius from smallest to largest. The electrons that are present in shells 1 and 2 are not involved in reactions and bonding.
How many are in Krypton Kr core electron and valence electrons. So electrons present in shells 1 and 2 are core electrons. That is the number of electrons in phosphorus is fifteen.
A neutral phosphorus atom has 15 total electrons. Due to the number of valence electrons Phosphorus is capable of forming three bonds with other elements. Oct 31 2010.
K Al B N. Phosphorus also has 15 electrons. The third shell is the outer valence shell so it has 5 valence electrons.
33P a beta-emitter 025 MeV with a half-life of 254 days. 34 2 Write the full electron configuration for selenium following the nl rule. The 10 remaining electrons from the first and second shells are core electrons.
Now Phosphorus electron configuration P 15 1s22s22p63s23p3 complete configuration. The orbit filling diagram and the electron dot diagram show the empty spaces for three more electrons and how there are. The highest-numbered shell is the third shell which has 2 electrons in the 3s subshell and 3 electrons in the 3p subshell.
The core electrons are those in the 1s 2s and 2p orbitals. That is a phosphorus atom has a total of fifteen electrons. An atom of phosphorus Z 15 has a total of 15 electrons.
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f. 1 how many valence electrons and how many core electrons does a neutral phosphorus atom have. The electrons will be placed in different orbitals according to the energy level.
The 10 remaining electrons from the first and second shells are core electrons. The ground state electronic configuration of phosphorus atom is 1s22s22p63s23p3 1 s 2 2 s 2 2 p 6 3 s 2 3 p 3. That is we can finally say that there are electrons equal to the atomic number in the phosphorus atom.
I am not sure what is meant by nl 3 write the electron configuration grouping electrons by their n. The highest-numbered shell is the third shell which has 2 electrons in the 3s subshell and 3 electrons in the 3p subshell. Therefore the number of core electrons present in ground state phosphorus atom is 10.
A periodic table also gives us the mass number of phosphorus the bigger number which is 31. The atomic number of phosphorusP is 15. Phosphorus-33 is composed of 15 protons 18 neutrons and 15 electrons.
The 10 remaining electrons from the first and second shells are core electrons. How many are in Phosphorus P core electron and valence electrons. A phosphorus atom has 10 core electrons.
Click to see full answer. Core How many core electrons does phosphorus have. There are 2 electrons at the first energy level and 8 at the second energy level.
Phosphorus is an element which is part of Group 15A neutral Phosphorus Atom has five valence electrons. It is used in life-science laboratories in applications in which lower energy beta emissions are advantageous such as DNA sequencing. It is in period 3 because it has orbits at 3 energy levels.
1 What is the atomic for selenium. When we write the configuration well put all 15 electrons in orbitals around the nucleus of the Phosphorus atom. That gives a total of 5 electrons so neutral phosphorus atoms have 5 valence electrons.
28 Votes That gives a total of 5 electrons so neutral phosphorus atoms have 5 valence electrons. Phosphorus has 15 electrons per atom. As you can see in the electron configuration the 3p sublevel has room for three more electrons.
This number is equal to protons neutrons in an atom so mass number protons neutrons. Therefore the phosphorus atom will have two electrons in the first shell eight in the 2nd orbit and five electrons in the 3rd shell. From the periodic table we see that the atomic number of phosphorus is 15.
Why is phosphorus P4.

Phosphorus Atom Good Example Earth Science Projects Science Project Models Kids Learning Tools

Phosphorus Electron Configuration Youtube

Phosphorus Valence Electrons Phosphorus Valency P With Dot Diagram
No comments for "How Many Core Electrons in Phosphorus"
Post a Comment